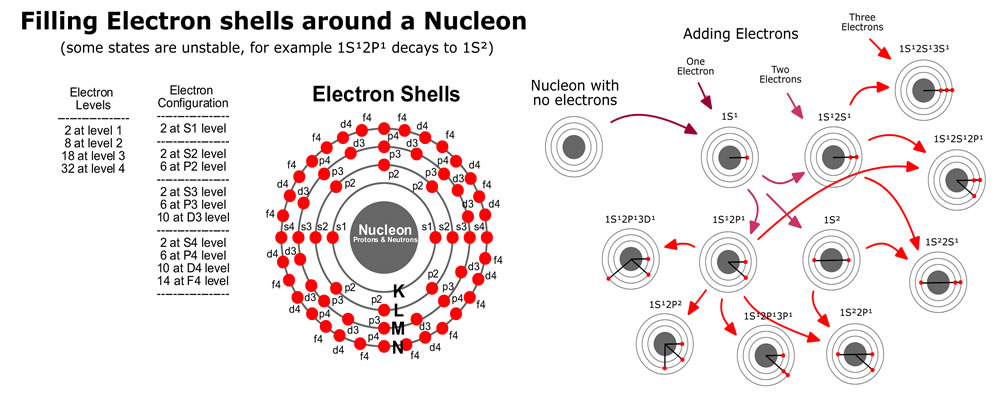
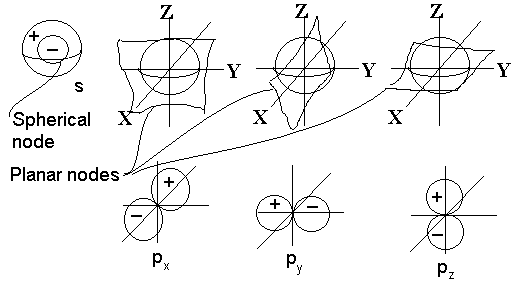
Before modelling protons and electrons under the coulomb force, a quick note about time scales and distance scales. Distances on the atomic scale for protons and atoms are often measured in picometers (10‾¹²), where the Bohr radius is 53pm. For electrons, distances can go as small as the Lorentz radius of 3 femtometers (10‾¹⁵). For tracking electrons, attoseconds (10‾¹⁸) is […]