What to do?
- Drag your cursor across the screen to aim the crosshairs.
- Click the “Shoot” button.
What will happen?
- An electron will come shooting out from the bottom. If you are a good shot, and you figure out where to aim, you can try to dislodge the electron trapped in the first energy level of a hydrogen atom.
- Successfully dislodge the hydrogen electron from the hydrogen atom and you will earn credits you can use towards advancing your knowledge or winning prizes!
Click to play “Shoot the Electron”
What you learn
- See what it looks like over very small timeframes with electrons and protons under the influence of the coulomb force.
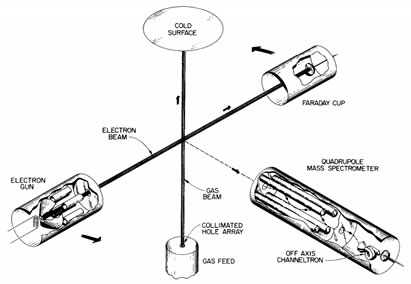
How big and how fast?
- It takes less then one femtosecond to dislodge an electron. Protons are shown with a Bohr (53 picometers) radius and electrons with a Lorentz (3 femtometers) radius.
- Protons weigh 1836 times more then electrons. Protons are tracked on a femtosecond timescale, but electrons, being thousands of times lighter and faster, are tracked on an attosecond timescale.
Real time animations
- Electrons dont just sit and wait for you to shoot, when disturbed they move around, aim carefully.
- Try adding energy to your electron. More energy means more speed.
- Pause/Play button to stop and start.
Click to play “Free the Proton”
Energy and Speed
- The energy of an electron is measured in evolts and speed in picometers per attoseconds. For reference, the speed of light is 300 picos per attosecond.
- Using the formula E=½mv²
10eV = 1.9 picos/attosec
100eV = 6.0 picos/attosec
500eV = 13.4 picos/attosec
 Now your a pro!
Now your a pro!
- The NIST maintains a database of ionization cross sections of molecules by electron impact, including H, He, and H². The cross sections are calculated using Binary-Encounter-Bethe (BEB) model.
- Their research shows that just under 100 eVolts of energy is best for knocking electrons out of hyrogen molecules, as the chart below illustrates.
The Hydrogen Molecule
- The five most common and stable chemical forms of hydrogen, are the cation (H+), anion (H-), dihydrogen (H2+), ortho and para hydrogen.
- It takes 13.6 eVolts of energy for a single electron to escape a single proton. This is called the ionization energy of hydrogen. It actually takes considerable more energy then 13.6 eVolts for an electron to dislodge another electron and get itself out of the way.
- Adding more energy to the electron does not always help. With more speed, more accuracy is required to deliver the proper glancing blow to dislodge the bonded electron and not get trapped.