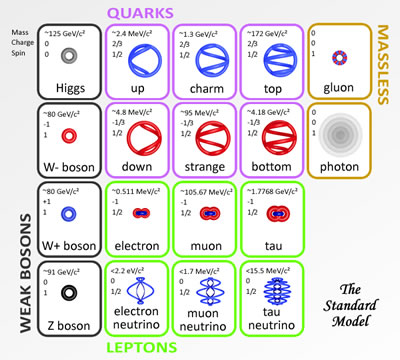
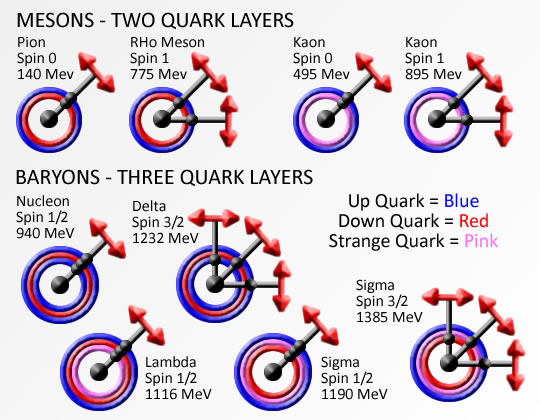
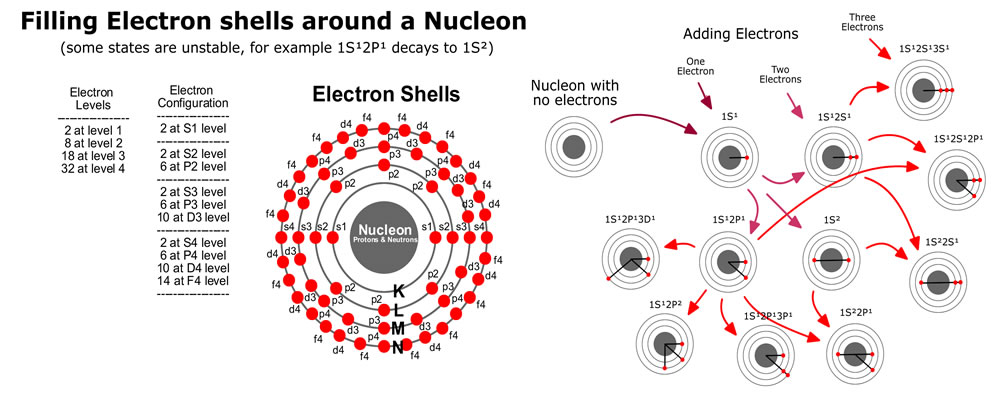
The base element of all matter is made up of protons, neutrons and electrons. Protons and neutrons bind together to form the all of the elements from hydrogen (called atoms), with 1 proton, to carbon with 12 protons, way up to highly unstable elements with over 100 protons. Electrons bind or stick to the atom with specific binding energies depending on the shell they attach to. Atoms are held together in molecules and structures when electrons bind to more then one close atoms.
Boyle's law from 1662, states that at constant temperature for a fixed mass, the absolute pressure and the volume of a gas are inversely proportional. This animation illustrates the normal air we breath at 20°C. Free flowing molecules are travelling an average 2.1 nanometers per picosecond, trapped in a 3D box, 30 x 10 x 7.5 nanometers in size.
In 1873, Johannes van der Waals, derived the Van der Waals equation where repulsion between molecules has the effect of excluding neighbors from a certain amount of space around each molecule. This principle together with the bonding between molecules is illustrated with liquid water molecules bonded together, trapped in a 3D box, 5 x 3 x 2 nanometers in size.
Explore the top of a block of potassium chloride, i.e. common table salt. Common table salt has a face centered cubic (fcc) structure or lattice. Enter a 3D perspective view from just above the surface of the solid. Drag a mouse across the screen to move forward, back or turn. You can see an overhead or topdown view of yourself in the top right-hand window.