Click to play “Shoot the Electron”
What to do?
- Drag your cursor across the screen to aim the crosshairs.
- Click the “Shoot” button.
What will happen?
- An electron will come shooting out from the bottom. If you are a good shot, and you figure out where to aim, you can try to dislodge the electron trapped in the first energy level of a hydrogen atom.
- Successfully dislodge the hydrogen electron from the hydrogen atom and you will earn credits you can use towards advancing your knowledge or winning prizes!
What you learn
- See what it looks like over very small timeframes with electrons and protons under the influence of the coulomb force.
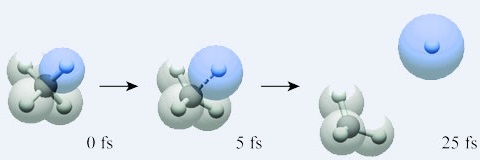
A Proton (hydrogen atom) floating
away from a Methane (CH4) Molecule
How big and how fast?
- It takes less then one femtosecond to dislodge an electron. Protons are shown with a Bohr (53 picometers) radius and electrons with a Lorentz (3 femtometers) radius.
- Protons weigh 1836 times more then electrons. Protons are tracked on a femtosecond timescale, but electrons, being thousands of times lighter and faster, are tracked on an attosecond timescale.
Real time animations
- Electrons dont just sit and wait for you to shoot, when disturbed they move around, aim carefully.
- Try adding energy to your electron. More energy means more speed.
- Pause/Play button to stop and start.
Click to play “Free the Proton”
Energy and Speed
- The energy of an electron is measured in evolts and speed in picometers per attoseconds. For reference, the speed of light is 300 picos per attosecond.
- Using the formula E=½mv², 10eV = 1.9 picos/attosec, 100eV = 6.0 picos/attosec, 500eV = 13.4 picos/attosec
Now your a pro!
- The NIST maintains a database of ionization cross sections of molecules by electron impact, including H, He, and H². The cross sections are calculated using Binary-Encounter-Bethe (BEB) model.
- Their research shows that just under 100 eVolts of energy is best for knocking electrons out of hyrogen molecules, as the NIST link shows.