|
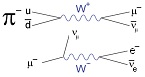
Pion Decay
|
The decay of the pion (π−) is due to the weak interaction.
The primary decay mode of a pion,
is a purely leptonic decay into a muon (μ−) and a muon antineutrino (ṽμ).
Soon after, the muon (μ−),
also through the weak interaction, decays into an electron (e-), a electron antineutrino (ṽe) and a muon neutrino (vμ).

|
Kaon Decay

|
The decay of a kaon (K+) into three pions (2 π+, 1 π−) is a process that involves both weak and strong interactions.
Weak interactions : The strange antiquark (s) of the kaon transmutes into an
up antiquark (ū) by the emission of a W+ boson; the W+ boson decays into a
down antiquark (đ) and an up quark (u).
Strong interactions : An up quark (u) emits a gluon (g) which decays
into a down quark (d) and a down antiquark (đ).
|
Stern-Gerlach

|
Electron spin detection.
This paper
and information
show how they shoot potassium atoms through about a .5cm channel of a 10cm long magnet and check
if they hit a 4mm paladium wire that is moved around having moved up or down due to spin.
An animation width of 1 meter is required with a timestep determined by
the speed of the potassium atom that depend on the oven temperature that drives the vapor pressure.
v = sqrt(3*RT/m)
R=boltzman constant
T=temperature (kelvin)
m=mass of a Potassium atom.
and speed variations based on Doppler broadening measurements:
50% in 1.67*c*sqrt(2RT/m)
97% in 3.34*c*sqrt(2RT/m)
"As a beginning graduate student back in 1923, I ... hoped with ingenuity and inventiveness
I could find ways to fit the atomic phenomena into some kind of a mechanical system... My hope to [do that]
died when I read about the Stern-Gerlach experiment... This convinced me once and for all that an ingenious
classical mechanism was out and that we had to face the fact that the quantum phenomena required a completely
new orientation."
Isidor I. Rabi (1898-1988)
|
|
Charge Radius
|
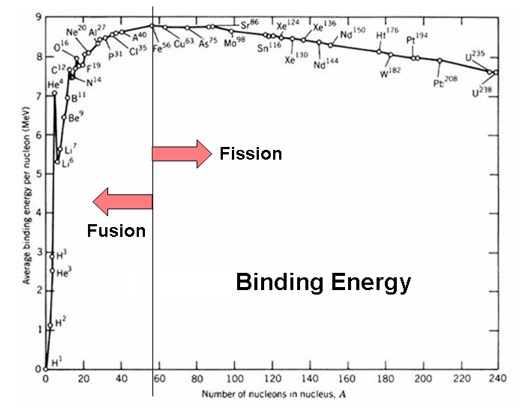
The proton charge radius is 0.8768 femtometers
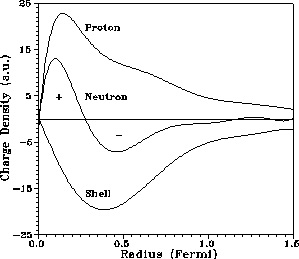
Studies have found an empirical relation between the charge radius and the mass number, A,
for heavier nuclei (A > 20):
R ≈ r0*A^⅓ where r0 is an empirical constant of 1.2–1.5 fm.
This gives a charge radius for the gold nucleus (A = 197) of about 7.5 fm.
Neutron and x-ray scattering cross-sections compared.
 Note that neutrons penetrate through Al much better then x-rays do, yet are strongly scattered by hydrogen.
Note that neutrons penetrate through Al much better then x-rays do, yet are strongly scattered by hydrogen.
|
Periodic Table

|
|
Photons and Matter
|
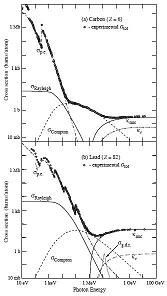
The passage of particles through matter, page 22 shows Photoelectric effect, Rayleigh scattering, Compton scattering, and pair production in a Nuclear or Electron field.
The animations compare the scatter profile of lead (Z=82) and carbon (Z=6) over a range of energy.
Rayleigh from 10ev to almost 1MeV for Carbon
Rayleigh from 10ev to over 10MeV for Lead
Compton 100ev to 1GeV
Pair production over 1MeV
Time and distance scales to be determined.
|
|
The Lamb Shift
|

Hydrogen energy levels are not quite exact.
"At the heart of the process is the exchange force by
which charges interact by the exchange of photons (the exchange force model of the electromagnetic force).
There can be a self interaction of the electron by exchange of a photon as sketched in the
Feynman diagram at left.
This "smears out" the electron position over a range of about 0.1 fermi
(Bohr radius = 52,900 fermis). This causes the electron spin g-factor to be slightly different
from 2. There is also a slight weakening of the force on the electron when it is very close to the
nucleus, causing the 2s electron (which has penetration all the way to the nucleus) to be slightly
higher in energy than the 2p(1/2) electron." (from Hyperphysics)
|
|
"Matter is built from particles under
the influence of forces"
|

|
1. Colliding Particles - Baryons, Mesons and Quarks
|
Protons and neutrons are in a class of particles called baryons, held together by the strong force,
with a mass of about 940 MeV. Both protons and neutrons are made of
three rotating up or down quark shells, attracted by the Casimir force,
with gluons acting as ball bearings.
|
|
|
Protons and neutron shells rotate in a coordinated manner creating spin 1/2 particles
(Bloch spheres are an SU(2) symmetry).
The Delta baryons are also made of up/down quarks and gluons
but do not have a coordinated spin producing 4 states (spin 3/2).
Lambda and Sigma baryons (up and/or down with a strange quark) vary only in how their spin is coordinated.
Lambda has 1 state (isospin 0) with 2 spin values (spin 1/2). Sigmas have 3 states (isospin 1)
where the up and down spin is either coordinated with 2 spin values (spin 1/2), or not coordinated with 4 spin values (spin 3/2).
|
|
When protons and neutrons are in high speed collisions, mesons (consisting of only two quarks) as well as baryons are created.
The pion has no spin (interlocked), while the rho meson has the same quarks with independent spin.
Mesons continue to decay into muons, electrons, gamma rays and neutrinos.
|
| Mesons - two quark layers |

π+ uđ
πº uū or dđ
π- ūd |

ρ+ uđ
ρº uū or dđ
ρ- ūd |
|
|

K+ uŝ
Kº dŝ or sđ
K- ūs |

K*+ uŝ
K*º dŝ or sđ
K*- ūs |
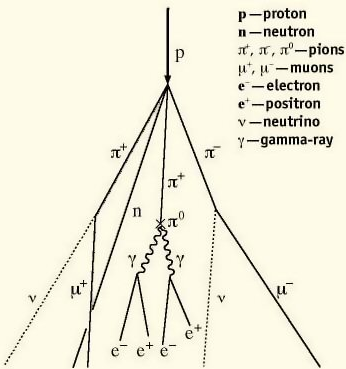
Cosmic Rays |
|
|
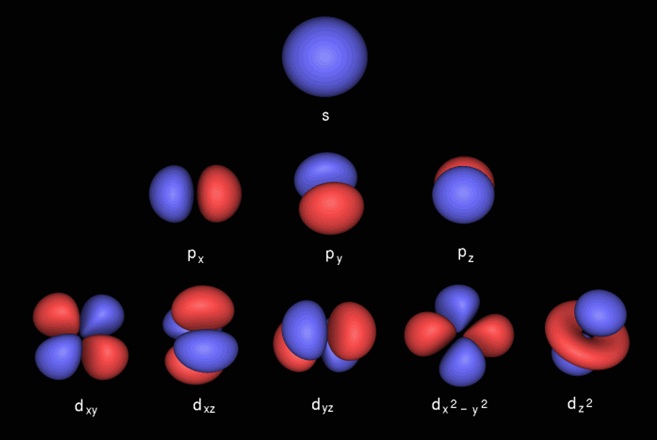
3. Energy Levels - Electron structure around the Nucleus
|
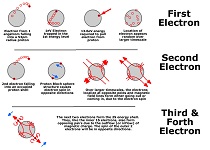
The nucleus and first four electrons
(click to view - 1S and 2S shells)
 |
|
|
Left alone, an electron falling into a proton, may hit the shell with enough kinetic energy to emit a photon, equal to the difference of energy inside and outside the nucleus.
This photon is called the K-alpha emission and helps to uniquely identify elements. If an electron is substantially turned or accelerated due to coulomb force, it may emit a
Bremsstrahlung (braking radiation) photon at various wavelengths.
If the electron hits the shell with more then the energy difference inside and outside the shell, two photons may be emitted in opposite directions. The sum of the two photon energies must equal the energy difference.
The emission of the photon results in the equivalent loss of the electrons kinetic energy (slows down), often trapping the electron inside the nucleus. This area is called the 1S shell and holds at most 2 electrons with opposite spins.
If an electron already exists inside the nucleon, then the electron drops to an even lower energy and emits a photon called the K-beta photon.
The energy of the photon emitted and the ionization energy holding the electron to the proton is calculated using Bohr, Ryberg and Moseley laws.
Only two electrons fit within this first energy level called the 1S level. In fact, the two electrons due to their repulsion and spin, will align themselves with the spin of the proton on opposite sides, forcing the magnetic charge of the electrons to be facing each other.
A third electron can enter this region due to its own kinetic energy and will emit a slightly higher energy
photon, in fact, this photon makes up the third in the "Lyman" series of emissions.
Additional energy levels are created through the interation of the nucleus and electrons, all of which are in motion.
|
|
4. Bonding - Elements, Electrons and Molecules
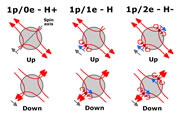
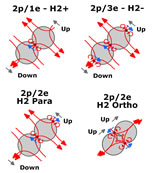
| Spin and Magnetic layout of Hydrogen & Bonded Hydrogen |
 |
|
 |
Bonding between elements occurs when an electron, trapped in an energy level of one element, gets trapped in an energy level of another element, thereby binding the two elements together.
Hydrogen consists of a proton and an electron and is neutral in charge. Although neutral, the proton acting as a shell of charge with the electron close to the edge of the shell,
will attract other neutral hydrogen atoms until one electron (or perhaps 2 in the ortho form) is trapped in both hydrogen shells binding them together.
The second element, helium, consists of two protons, for a charge of plus two. Helium is very stable with two electrons in
the first shell. This makes the first shell full and it will not accept another electron. Some compounds of helium will develop, for example He²+ or HeH+, but only where there is a shortage of electrons, as in a plasma.
Lithium has three protons with the third electron
attracted to the nucleus, but repelled by the two electrons in the first shell.
Lithium atoms can pair together, to form dilithium, where a pair of electrons with
opposite spin reside between the two atoms.
Lithium binds with itself to form a cubic
body centered metal.
Lithium and hydrogen bind in a cubic structure.
Beryllium has four protons and binds into a strong hexagonal close packed structure.
Beryllium also binds with hydrogen to form beryllium hydride (BeH2).
Boron with five protons does not form an easy to define grid. As a solid it exists mostly in a
icosahedron structure of 12 boron atoms. These icosahedrons can be bound together in various
different forms. It also exists as a gas combined with hydrogen called diboron (B6H6) with
two of the hydrogen atoms between the two boron nucleons.
|
|
Simple hydrocarbons showing outer
shells and inner structure |
 |
|
| Organic Molecules |
 |
5. Hydrocarbons and Organic Molecules
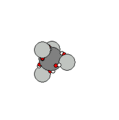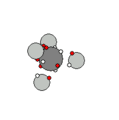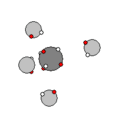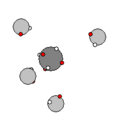
Carbon & Methane |
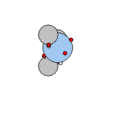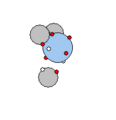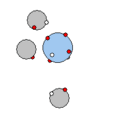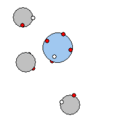
Nitrogen & Ammonia |
Oxygen & Water |
Carbon has six protons and together with the electron shells, form a very stable structure that easily
binds with other carbon nucleons and hydrogen to form hydrocarbons. In addition to hydrocarbons, carbon binds with itself in a diamond, graphic or graphene sheet solids.
The next two elements, nitrogen and oxygen, together with the hydrocarbons build the basic building blocks of life.
Carbon has four bonding spots, nitrogen has three and oxygen has two, allowing for the construction of large molecular structures.
Hydrocarbons consist of long chains of carbon and hydrogen where two of the bonds are for the hydrogen chain and some hold hydrogen to each carbon.
Alcohols have a single bonded oxygen attached together with a companion hydrogen. Sugars integrate the oxygen into a carbon loop with two single bonds. Acids contain the alcohol bond together with a double bonded oxygen,
and finally, the amino acids include a strategically placed nitrogen just beside the acid structure.
The lipids form natural cell walls and the amino acids are strung together to form peptides, proteins and eventually, cells themselves, both plant and animal.
|
|
6. Learn more
Animated Physics software allows you to model any grid size using many types of common
objects. Animations are built to a specific timescale and forces such as charge and gravity
can be applied to the objects and the animations run to see the results.
The laws of Animated
Physics
(a computer model of Matter and Energy)
Download Software
Animated Physics Memories software can be downloaded here. You will be able to view and create animations with this software.
Click below to join and learn more.
Join NOW!
|
|
|
If you build it..
It will come to life. |
These are the kind of animations you can watch or do yourself.
All of the animations shown here are prebuilt for you and will
run on a click of a button.
The easy to follow, tutorial style
of the animations, make it easy to learn how to manipulate the
animations. In no time at all, you can be moving objects around,
changing the speed and properties of things and adding in new objects.
In effect, start building your own experiments. |
| Think Big: |
Amimations already done like the Space Station orbit in real time with Height = 360 km, Speed = 7.69 km/sec
and watch it orbit the earth every 90 minutes.
Try out the Earth/Moon system
and see Height = 399630 km, Speed = 960.16 km/sec, with an orbit of about once per month.
Free software and support if you will help us build these. What we need:
Mars and its moons
Saturn and moons...
On a much bigger scale, the location of our neighborhood stars needs to be plotted in 3D. Our galaxy, the milky way, needs a 3D structure.
Contact us by email for info on how you can help.
|
| Talking to Bohr in 1933,
Einstein introduced an experiment
to question the uncertainty principle |
Suppose two particles are set in motion towards each
other with the same, very large, momentum, and that
they interact with each other for a very short time when
they pass at known positions.
Consider now an observer
who gets hold of one of the particles, far away from the
region of interaction, and measures its momentum; then,
from the conditions of the experiment, he will obviously
be able to deduce the momentum of the other particle.
If, however, he chooses to measure the position of the first
particle, he will be able to tell where the other particle is.
This is a perfectly correct and straightforward deduction
from the principles of quantum mechanics; but is it not
very paradoxical? How can the final state of the second
particle be influenced by a measurement performed on
the first, after all physical interaction has ceased between
them?
|
| Think Small: |
Tiny charge simulations involving the electrons and protons, the charge
(coulomb) force over distances of less then a picometer and timescales
in the attoseconds.
Light travels at the speed of 300 picometers per
attosecond and shoots past a proton in less then an attosecond. Most
electrons travel considerably slower with a speed in the range of 2 picometers
per attosecond.
Watch heavier and more sluggish protons travel a much
slower relative speed due to their weight. Track multiple particles of
various charges and weights and watch how molecules form in real time. |

Electricity and Magnetism |
The Electron
 |
|
The motion of free electrons are controlled by the structure of the nearby
nucleons and electrons.
The Lorentz force is the force on an electron due to electromagnetic
fields.
It is given by the following equation in terms of the electric and magnetic fields:
F = q[E + (v x B)], where F is the force (in newtons), E the electric field
(volts per meter), B is the magnetic field (in teslas), q the electric charge and
v is the velocity (meters/second).
An electron will "precess" in a magnetic field causing its "average" direction to be perpendicular to the magnetic field.
Due to the precession, the electron will go in one of only two directions (up or down) in a magnetic field such as a Stern-Gerlach experiment.
|
| Electron Drift Velocity |
The speed of electon drift for a copper wire
with a diameter of 1 mm, and a current I=3 amperes, can be calculated
to be 0.0003 m/s, a very slow 1.0 m/hour.
The big wire cross-section compared to the very
small copper nucleon & electron grid size means that although the electrons are going by
at slow speeds, individual electrons are jumping from one grid spot
to another using much faster speeds approaching 1000.0 m/s.
|
| Electrons and Bell's Inequality |
Decaying particles
can produce pairs of electrons spinning and travelling in opposite directions.
 When observers of the two electrons (usually refered to as Bob and Alice), check the spin,
they seem to find many more electrons with spin aligned then expected.
When observers of the two electrons (usually refered to as Bob and Alice), check the spin,
they seem to find many more electrons with spin aligned then expected.
When two devices are aligned in matching orientations,
100% of the results will match no matter the orientation of the devices.
If either device is turned to 45° from the other, a suprisingly large 85% of
results match and 15% differ.

The explaination lies in the structure of the electron. The electron does not allow its spin to be determined
when close to 90°. Electrons start to tumble and do not make it to the detector (red), thereby skewing the statistical results.
The green area shows the relationship of spin between the two
observers. The missing particles are usually assigned "the fair-sampling assumption". We use the missing electrons to reveal properties of the electron.
|
|







 When observers of the two electrons (usually refered to as Bob and Alice), check the spin,
they seem to find many more electrons with spin aligned then expected.
When observers of the two electrons (usually refered to as Bob and Alice), check the spin,
they seem to find many more electrons with spin aligned then expected.